GenBio Inc. Digging Deep into Inflammatory Diseases
Chronic inflammation: Cause of more than half of deaths worldwide
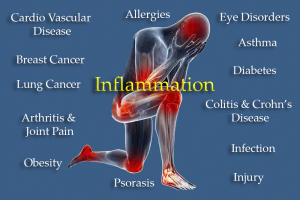
ALISO VIEJO, CA, UNITED STATES, May 21, 2025 /EINPresswire.com/ -- Inflammation is the physiological process of releasing specific chemicals to fight infections and heal damaged tissues. If this release of chemicals continues after the injury or infection is healed, inflammation becomes a pathological process, decreasing the function of the cells in the body. As an example, obesity is now defined as a chronic low-grade inflammation where the release of adipokines, a complex group of pro- and anti-inflammatory cytokines from expanding adipocytes in visceral adipose tissue, initiates and sustains cell damage throughout the body. Increased low-grade inflammation is associated with diets with increased highly processed foods, saturated fatty acids, simple sugars and salt and low in dietary fibre and vitamins. Digestion of food takes place in the intestines with nutrients mainly absorbed in the small intestine and water in the large intestine.
The intestines contain a complex community of bacteria known as the gut microbiota which is essential for fermentation of food and protection against pathogens. The gut microbiota allow the conversion of complex carbohydrates, not digested by the host, to short-chain fatty acids such as butyrate which are absorbed and may be anti-inflammatory. The gut microbiota also regulate the permeability of the intestines with some species promoting a “leaky gut”. This allows microbial metabolites and components of the bacteria, such as lipopolysaccharides, to enter the circulation and start an inflammatory reaction by the release of cytokines. As increased inflammation is associated with many diseases such as cardiovascular disease, diabetes, obesity, inflammatory bowel disease and cancer, understanding the role of the gut microbiota in these diseases is important. In obesity, increased inflammatory responses occur with pro-inflammatory macrophages accumulating in adipose tissue, possibly with hypoxia as the initiating event leading to greater expression of hypoxia-inducible factor 1-α and increased inflammatory responses in the liver, pancreatic islets and gastrointestinal tract. Adipose tissue inflammation decreases remote organ function, considered causative for the complications of obesity throughout the body, including the liver, heart, blood vessels, brain, fat pads and kidneys.
The hypothalamus is a part of the brain with a vital role in regulation of energy balance in the body. Chronic inflammatory activation of glial cells in the hypothalamus leads to changes in feeding habits, production of heat and adipokine signalling, leading to metabolic disorders. Further, maternal obesity and inflammation may lead to metabolic reprogramming in the foetus, which could influence both childhood and adult body weight and composition, thus increasing the risk of transgenerational Inheritance of obesity susceptibility.
Effective anti-inflammatory compounds include the anthocyanins, active molecules in purple fruits such as berries and plums. The anthocyanins regulate pro-inflammatory cytokines in both healthy and chronic disease states. There are many mechanisms for the anti-Inflammatory effects of anthocyanins that could be applicable in chronic inflammatory diseases, including inhibiting the release of pro-inflammatory factors, reducing TLR4 expression, inhibiting the NF-κB and MAPK signalling pathways, and reducing the production of NO, ROS, and prostaglandin E2. Reducing inflammation throughout the body may play a role in anthocyanin-induced benefits in chronic disease, including obesity, but extensive clinical validation is still required.
The gut microbiota and brain are connected by a network of nerves that communicate in both directions to Maintain homeostasis in the gastrointestinal tract and brain. Dysbiosis of the gut microbiota may allow the progression of both systemic inflammation and neuroinflammation. Thus, much recent research has focused on the relationships between gut microbiota and neurological disorders such as schizophrenia and autism spectrum disorder as well as neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and ischaemic stroke, which involve the death of vulnerable populations of neurons.
Mediators of neuroinflammation include microglia, astrocytes and oligodendrocytes as well as damaged and dysfunctional mitochondria. Anthocyanins and their metabolites produce neuroprotective activities by decreasing neuroinflammation, preventing excitotoxicity, preventing aggregation of proteins, activating pro-survival pathways while inhibiting pro-apoptotic pathways and improving axonal health.
The wide range of potential mechanisms that anthocyanins, such as cyanidin glucoside, may activate to prevent or overcome neurotoxicity includes suppression of c-Jun N-terminal kinase activation, amelioration of cellular degeneration, activation of the brain-derived neurotrophic factor signalling and restoration of Ca 2+ and Zn 2+ homeostasis. Blueberry extract (150 mg/kg/day) containing anthocyanins promoted neuronal autophagy to decrease neuronal damage in transgenic APP/PS1 mice with mutations associated with early-onset Alzheimer’s disease. Further, protocatechuic acid may be the major metabolite producing neuroprotection. After three months ' treatment with tart cherries containing anthocyanins and other polyphenols in middle-aged adults, possible changes in amino acid metabolism have been suggested as the mechanism of improved attention, feelings of alertness, and mental fatigue. These changes could be beneficial if they can be translated to preventing or treating neurological or neurodegenerative diseases by dietary changes in humans.
Thus, the effectiveness of anthocyanins and their metabolites in existing neurodegenerative diseases in humans, where significant neuronal loss has already occurred, is uncertain but plausible. Therefore, targeting neuroinflammation with anthocyanins is a promising strategy in treating neurological disease, although translating results from animal models to patients with neurological disease requires further research. Further, direct pharmacological actions in the brain of orally administered anthocyanins require that anthocyanins pass through the blood–brain barrier. Yet, the permeability of anthocyanins is low. This suggests that the primary reason for decreased neurotoxicity with anthocyanins could be that changes in gut microbiota lead to reduced neuroinflammation.
Todd D. Sonoga
GenBio Inc.
+1 949-705-8021
email us here
Visit us on social media:
LinkedIn
Facebook
X
GenBio Inc Elevator Pitch
Distribution channels: Banking, Finance & Investment Industry, Culture, Society & Lifestyle, Healthcare & Pharmaceuticals Industry, Science, World & Regional
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release